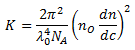Dynamic Light Scattering (DLS) with the Malvern Zetasizer Nano-ZS
The Zetasizer Nano ZS from Malvern can detect three different particle characteristics in a liquid medium – the particle size, the zeta potential and the molecular weight – using the principles of light scattering.
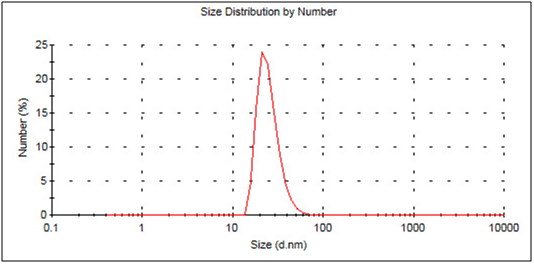
Particle size
The measurement of the particle size in this DLS (dynamic light scattering) analyzer bases on detecting the Brownian movement of the particles in the sample. This movement can be set in relation to the particle size. Practically, the measurement occurs by irradiation the sample with a laser. Based on light scattering an interference pattern of light and dark points gets generated on the detector window. Small particles have a higher mobility (Brownian motion) and therefore cause a faster fluctuation of brightness points on the screen. Based on the rate of these fluctuations the particle size can get calculated. The relationship between particle size and Brownian motion is described by the Stokes-Einstein-relation:
| with | D | = | Diffusion constant |
| k | = | Boltzmann constant | |
| T | = | absolute temperature | |
| η | = | dynamic viscosity of the solvent | |
| r | = | Particle radius |
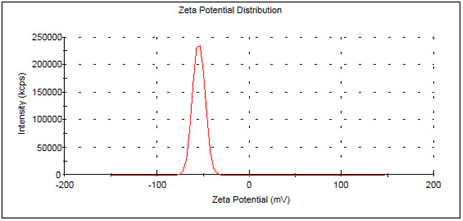
Zeta potential
On the surface of charged particles a layer of counter ions exists – the so-called Stern layer with the Stern potential. The counter ions are bound strongly to the surface of the charged particle. Another more diffuse layer of charged particles follows, in which a stable conformation forms. Within this boundary layer, the ions move with the movement of the whole particle in the same direction. Outside the boundary this directed movement does not exist. Hence the boundary is also the shear surface of the particle to the surrounding liquid. The potential at this boundary layer is called zeta potential. The described two layers are called the electrochemical double layer.
The measurement of the zeta potential is performed with the Zetasizer Nano indirectly by measuring the electrophoretic mobility by laser Doppler velocity measurements in a special cell. Again the light is scattered by the particles and fluctuations of the light intensity at the detector, which are a function of the particle velocity, get recorded.
Practically the measurement is carried out by applying a defined voltage to two electrodes in the cell leading to different speeds of the particles. Particles with higher potential have a higher speed in the electrical field.
The zeta potential can be calculated with the Henry function.
| with | UE | = | Electrophoretic mobility |
| ε | = | Dielectric constant | |
| Z | = | Zeta potential | |
| f(Ka) | = | Henry Funkcion (for aqueous systems, a value of 1.5 is used. For small particles in low dielectric constant media a value of 1 is used.) | |
| η | = | viscosity |
The zeta potential is the key number for the stability of dispersions. Due to the mutual repulsion of charged particles, dispersions with a zeta potential above 30 mV or below -30 mV are classified as stable with no tending to flocculation.
Additionally there is a strong dependence of the zeta potential on the pH. By the addition of alkalis or acids the zeta potential can be increased or decreased depending on the starting point. For example can particles with a negative zeta potential by adding acid be changed to the isoelectric point and beyond. The isoelectric point represents the unstable point of a dispersion, where the zeta potential is zero.
Molecular weight
The molecular weight can be measured with the Zetasizer Nano using static light scattering (SLS). Again, a light scattering in all directions is generated by irradiating the sample by a laser light source. In contrast to the measurements of particle size and zeta potential, where brightness fluctuations were detected, this time the time averaged intensity of the scattered light gets detected. The intensity of the scattered light at a defined angle of a molecule is proportional to the product of the average molecular weight and the concentration of the molecules. Practically, the light intensity is detected at a certain angle at different sample concentrations in a defined time. By using Rayleigh’s equation
| with | Rθ | = | Rayleigh ratio of scattered light to incident light |
| M | = | Molecular weight of the sample | |
| A2 | = | Second virial coefficient | |
| P(θ) | = | Angular dependence of light scattering | |
| K | = | Optical constant |
| with | NA | = | Avogadro constant |
| λ0 | = | Wave length of the laser source used | |
| n0 | = | Refractive index oft he solvent used | |
| dn/dc | = | Refractive index increment |
and plotting the intensity against the concentration oft he scatters light (Debye plot) the molecular weight can be calculated. The extrapolation of the straight line of the intersection with the y axis (for x = 0) represents the reciprocal of the molecular weight.
With the Zetasizer Nano molecular weights of a few hundred g/mol to 500,000 g/mol for linear polymers and 20,000,000 g/mol for almost spherical polymers and proteins are detectable.