Publications
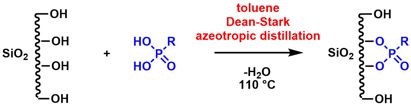
[60] Straightforward Immobilization of Phosphonic Acids and Phosphoric Acid Esters on Mesoporous Silica and their Application in an Asymmetric Aldol Reaction. Weinberger, C.; Heckel, T.; Schnippering, P; Schmitz, M.; Guo, A.; Keil, W.; Marsmann, H.C.; Schmidt, C.; Tiemann, M.; Wilhelm, R. Nanomaterials 2019, 9, 249-260.
[59] Determination of the Refractive Indices of Ionic Liquids by Ellipsometry, and their Application as Immersion Liquids. Wu, X.; Muntzeck, M.; De Los Acros, T; Grundmeier, G.; Wilhelm, R.; Wagner, T. Appl. Opt. 2018, 57, 9215-9222.
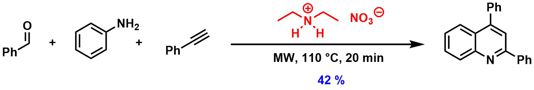
[58] Protic Ionic Liquids as Catalysts for a Three-Component Coupling/Hydroarylation/Dehydration Tandem Reaction. Muntzeck, M.; Wilhelm, R. Z. Naturforsch. 2018, 73b, 515–519.
[57] Graphene Oxide as Flexibilizer for Epoxy Amine Resins. Wolk, A.; Rosenthal, M.; Weiß, J.; Voigt, M.; Wesendahl, J.; Hartmann, M.; Grundmeier, G.; Wilhelm, R.; Meschut, G.; Tiemann, M.; Bremser, W. Prog. Org. Coat. 2018, 122, 280-289.
[56] A Novel Lubricant Based on Covalent Functionalized Graphene Oxide Quantum Dots. Wolk, A.; Rosenthal, M.; Neuhaus, S.; Huber, K.; Brassat, K.; Lindner, J. K. N.; Grothe, R.; Grundmeier, G.; Bremser, W.; Wilhelm, R. Scientific Reports 2018, 8, 5843.
[55] A Camphor Based 1,3-Diamine Ru(II) Terpyridine Complex: Synthesis, Characterization, Kinetic Investigation and DNA Binding. Milutinović, M. M.; Bugarčić, Z. D.; Wilhelm, R. New. J. Chem. 2018, 42, 7607-7611.
[54] New Pyridinium Based Ionic Dyes for the Hydrogen Evolution Reaction. Konieczna, D. D.; Biller, H.; Witte, M.; Schmidt, W. G.; Neuba, A.; Wilhelm, R. Tetrahedron 2018, 74, 142-149.
[53] Reactivity of Grubbs-Hoveyda II Complexes including Extended N-Heterocyclic Carbenes with a Bicyclic Camphor-Based Framework. Rais, E.; Flörke, U.; Wilhelm, R. Synthesis 2017, 49, 2852-2864.
[52] A Sophisticated Approach towards a New Class of Copper(I)-Sulfur Cluster Complexes with Imidazolinium-Dithiocarboxylate Ligands. Ortmeyer, J.; Flörke, U.; Henkel, G.; Wilhelm, R.; Neuba, A. Eur. J. Inorg. Chem. 2017, 3191-3197.
[51] The Use of Stable Carbene-CO2 Adducts for the Polymerization of Trimethylene Carbonate. Reitz, A. K.; Sun, Q.; Wilhelm, R.; Kuckling, D. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 820-829.
[50] Synthesis and Investigation of New Cyclic Haloamidinium Salts. Rais, E.; Flörke, U.; Wilhelm, R. Z. Naturforsch. 2016, 71b, 667-676.
[49] Straightforward Diastereoselective Synthesis of P-chirogenic (1R)-1,8,8-Trimethyl-2,4-diaza-3-phosphabicyclo[3.2.1]octane 3-Oxides. Application as Chiral NMR Solvating Agents. Uzarewicz-Baig, M.; Wilhelm, R. Heteroatom Chem. 2016, 27, 121-134.
[48] Influence of Ionic Liquids on an Iron(III) Catalyzed Three-Component Coupling/Hydroarylation/Dehydrogenation Tandem Reaction. Muntzeck, M.; Wilhelm, R. Int. J. Mol. Sci. 2016, 17, 860-868.
[47] Chiral Imidazolinium Salts with TIPS Groups for the Palladium Catalyzed alpha-Arylation and as Chiral Solvating Agents. Gilani, M. A.; Rais, E.; Wilhelm, R. Synlett 2015, 26, 1638-1641.
[46] Crystal Structure of Tris(1,3-dimesityl-4,5-dihydro-1H-imidazol-3-ium) tetrabromidocobaltate(II) bromide chloroform hexasolvate. Rais, E.; Flörke, U.; Wilhelm, R. Acta Cryst. 2015, E71, m177-m178.
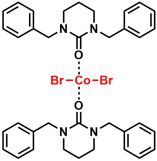
[45] Crystal Structure of Dibromidobis(1,3-dibenzyl-1,3-diazinan-2-one-κO)cobalt(II). Rais, E.; Flörke, U.; Wilhelm, R. Acta Cryst. 2015, E71, m160-m161.
[44] Crystal structures of diiodidobis[(1S,5S )-4-mesityl-1,2,8,8-tetramethyl-2,4-diazabicyclo[3.2.1]octan-3-ylidene-κC3]palladium(IV) and dichlorido[(1S,5S )-4-mesityl-1,2,8,8-tetramethyl-2,4-diazabicyclo[3.2.1]octan-3-ylidene-κC3](triphenylphosphane-κP )palladium(IV). Rais, E.; Flörke, U.; Wilhelm, R. Acta Cryst. 2015, E71, 919-922.
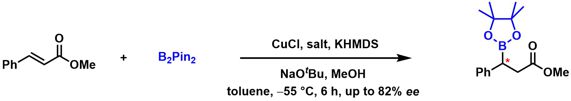
[43] Synthesis of New Camphor-Based Carbene Ligands and Their Application in a Copper Catalyzed Michael Addition with B2Pin2. Koppenwallner, M.; Rais, E.; Uzarewicz-Baig, M.; Tabassum, S.; Gilani, M. A.; Wilhelm, R. Synthesis 2015, 47, 789-800.
[42] Synthesis of New Copper(I) Based Linear 1-D-Coordination Polymers with Neutral Imidazolinium-Dithiocarboxylate Ligands. Neuba, A.; Ortmeyer, J.; Konieczna, D. D.; Weigel, G.; Flörke, U.; Henkel, G.; Wilhelm, R. RSC Adv. 2015, 5, 9217-9220.
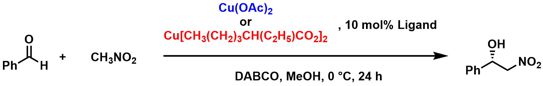
[41] Highly Regioselective Synthesis of Chiral Diamines via a Buchwald-Hartwig Amination from Camphoric Acid and their Application in the Henry Reaction. Uzarewicz-Baig, M.; Koppenwallner, M.; Tabassum, S.; Wilhelm, R. Appl. Organometal. Chem. 2014, 28, 552-559.
[40] Investigation of Imidazol(in)ium-dithiocarboxylates as Sensors for the Detection of Mercury(II) and Silver(I) Ions. Konieczna, D. D.; Blanrue, A.; Wilhelm, R. Z. Naturforsch. 2014, 69b, 596-604.
[39] Ring Opening Polymerization of Organic Carbonates Using CO2-Carbene Adducts as Effective Organocatalyst. Reitz, A.; Wilhelm, R.; Kuckling D. Macromol. Symp. 2013, 334, 92-97.
[38] Chiral Ionic Liquids Based on Nicotine for the Chiral Recognition of Carboxylic Acids. Heckel, T.; Winkel, A.; Wilhelm, R. Tetrahedron: Asymmetry 2013, 24, 1127-1133.
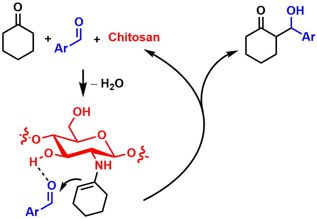
[37] An Ionic Liquid Solution of Chitosan as Organocatalyst. Heckel, T.; Konieczna, D. D.; Wilhelm, R. Catalysts 2013, 3, 914-921.
[36] Invited book chapter: Non-covalent Activation - Lewis Acids. Heckel, T.; Wilhelm, R. in Comprehensive Enantioselective Organocatalysis, ed. Dalko, P. I.; Wiley-VCH, Weinheim, 2013, p.413.
[35] Imidazolinium and Amidinium Salts as Lewis Acid Organocatalysts. Sereda, O.; Clemens, N.; Heckel, T.; Wilhelm, R. Beilstein J. Org. Chem. 2012, 8, 1798-1803.
[34] Imidazolinium Sulfonate and Sulfamate Zwitterions as Chiral Solvating Agents for Enantiomeric Excess Calculation. Tabassum, S.; Gilani; M.; Wilhelm, R. Tetrahedron: Asymmetry 2011, 22, 1632-1639.
[33] New Chiral Ionic Liquids Based on Enantiopure Sulphate and Sulfonate Anions for Chiral Recognition. Winkel, A.; Wilhelm, R. Eur. J. Org. Chem. 2010, 5817-5824.
[32] Invited Review: Lewis Acid Organocatalysts. Sereda, O.; Tabassum, S.; Wilhelm, R. Top. Curr. Chem. 2010, 291, 349-393.
[31] New Chiral Ionic Liquids Based on Imidazolinium Salts. Winkel, A.; Wilhelm, R. Tetrahedron: Asymmetry 2009, 20, 2344-2350.
[30] New Enantiopure NHCs Derived from Camphor. Reddy, P. V. G.; Tabassum, S.; Blanrue, A.; Wilhelm, R. Chem. Commun. 2009, 5910-5912.
[29] Hindered Brønsted Bases as Lewis Base Catalysts. Tabassum, S.; Sereda, O.; Reddy, P. V. G.; Wilhelm, R. Org. Biomol. Chem. 2009, 7, 4009-4016.
[28] Enantiopure Imidazolinium Dithiocarboxylates as Highly Selective Novel Organocatalysts. Sereda, O.; Blanrue, A.; Wilhelm, R. Chem. Commun. 2009, 1040-1042.
[27] Methylated Imidazolinium-Dithiocarboxylates: Two Representatives of a New Class of Ionic Liquids. Blanrue, A.; Wilhelm, R. Synthesis 2009, 583-586.
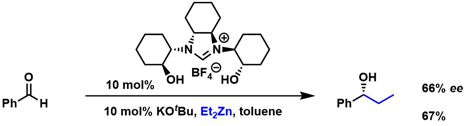
[26] New Enantiopure Imidazolinium Carbene Ligands Incorporating Two Hydroxy Groups for Lewis Acid Catalyzed Diethyl Zinc Addition to Aldehydes. Gilani, M.; Wilhelm, R. Tetrahedron: Asymmetry 2008, 19, 2346-2352.
[25] Influence of the Substitution Pattern of Cp-Iron-Arene Salts in the Solid-State Synthesis of New Carbon Nanostructures. Winkel, A.; Jain, D.; Wilhelm, R. Organometallics 2008, 27, 3430-3434.
[24] Invited Review: Recent Advances in the Synthesis and Application of Chiral Ionic Liquids. Winkel, A.; Reddy, P. V. G.; Wilhelm, R. Synthesis 2008, 999-1016.
[23] Hexamethyldisilazane Sodium Salt as Highly Active Lewis Base Catalyst for the Staudinger Reaction. Sereda, O.; Wilhelm, R. Synlett 2007, 3032-3036.
[22] Invited Paper: Solid-State Synthesis of Carbon-Nanostructures. Wilhelm, R.; Winkel, A.; Jain, D. J. Fudan University (Natural Science) 2007, 46, 701-702.
[21] An Easy Way to Produce a-Iron Filled Multiwalled Carbon Nanotubes. Jain, D.; Wilhelm, R. Carbon 2007, 45, 602-606.
[20] Easy Accessible Chiral Bis-Hydroxy Imidazolinium Salts as Shift Reagents and Carbene Precursors. Jurčík, V.; Gilani, M.; Wilhelm, R. Eur. J. Org. Chem. 2006, 5103-5109.
[19] Preparation of New Enantiopure Imidazolinium Salts and Their Evaluation as Catalysts and Shift Reagents. Jurčík, V.; Wilhelm, R. Tetrahedron: Asymmetry 2006, 17, 801-810.
[18] Unexpected Behaviour of Tosylated and Acetylated Imidazolinium Salts. Clemens, N.; Sereda, O.; Wilhelm, R. Org. Biomol. Chem. 2006, 4, 2285-2290.
[17] Solid-State Synthesis of Well Defined Carbon Nanocapsules from Organometallic Precursors. Jain, D.; Winkel, A.; Wilhelm, R. Small 2006, 2, 752-755.
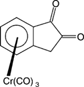
[16] Synthesis of Enantiopure Tricarbonyl(indan-1,2-dione)chromium. Leinweber, D.; Weidner, I.; Wilhelm, R.; Wartchow, R.; Butenschön, H. Eur. J. Org. Chem. 2005, 5224-5235.
[15] An Imidazolinium Salt as Ionic Liquid for Medium and Strong Bases. Jurčík, V.; Wilhelm, R. Green Chem. 2005, 7, 844-848.
[14] Imidazolinium Salts as Catalysts for the aza-Diels-Alder Reaction. Jurčík, V.; Wilhelm, R. Org. Biomol. Chem. 2005, 3, 239-244.
[13] Imidazolinium-Carbodithioate Zwitterions as Organocatalysts for the Cyanosilylation of Aldehydes. Blanrue, A.; Wilhelm, R. Synlett 2004, 2621-2623.
[12] Preparation of Aminals in Water. Jurčík, V.; Wilhelm, R. Tetrahedron 2004, 60, 3205-3210.
[11] Near-Quantitative Solid-State Synthesis of Carbon Nanotubes from Homogeneous Diphenylethynecobalt and -Nickel Complexes. Iyer, V. S.; Vollhardt, K. P. C.; Wilhelm, R. Angew. Chem. 2003, 115, 4515-4519; Angew. Chem. Int. Ed. 2003, 42, 4379-4383.
[10] Palladium Catalysed Suzuki Reactions of Fluoroarenes. Widdowson, D. A.; Wilhelm, R. Chem. Commun. 2003, 578-579.
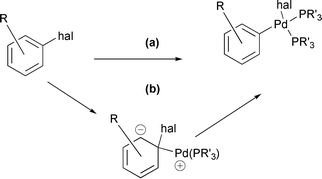
[9] A Computational Study of the Mechanism of Palladium Insertion into Alkynyl and Aryl Carbon–Fluorine Bonds. Jakt, M.; Johannissen L.; Rzepa, H. S.; Widdowson, D. A.; Wilhelm, R. J. Chem. Soc., Perkin Trans. 2 2002, 576-581.
[8] The Tricarbonylchromium Complex of a Trimethyltin-Substitued N‑(Triisopropylsilyl)indole - A Dynamic NMR Study of Multiple Independent Rotation Processes in the Solid State with an X-ray Diffraction Structure and Molecular Mechanics Calculations. Aliev, A. E.; Anderson, J. E.; Butler, D.; Gonzalez-Quteirino, J.; Lunazzi, L.; Mazzanti, A.; Steed, J. W.; Wilhelm, R. Eur. J. Inorg. Chem. 2002, 133-140.
Abstract: The title compound was studied by dynamic NMR spectroscopy in solution and in the solid state, and by MM3 molecular mechanics calculations of model compounds. Barriers to rotation of the trimethyltin group, the tricarbonylchromium group, the triisopropylsilyl group, and the isopropyl groups were all too small to measure by NMR in solution i.e. less than about 5 kcal/mol. All of these barriers could be measured, however, in the solid state, are of different sizes, and are surprisingly large, up to 12.9 kcal/mol. A crystal structure determination shows close intermolecular interactions of these triple rotors that explain these high rotation barriers.
[7] Dilithiation of Arenetricarbonylchromium(0) Complexes with Enantio-selective Quench: Application to Chiral Biaryl Synthesis. Tan, Y.-L.; White, A. J. P.; Widdowson, D. A.; Wilhelm, R.; Williams, D. J. J. Chem. Soc., Perkin Trans. 1 2001, 3269-3280.
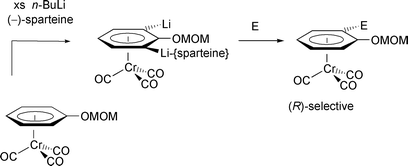
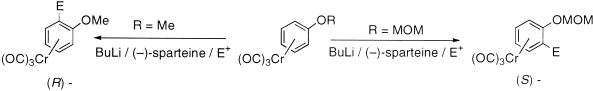
[6] Reversal of Asymmetric Induction in Arenetricarbonylchromium(0) Complexes via Dilithiation with the (–)-Sparteine / BuLi System and Enantioselective Quench. Tan, Y.-L.; Widdowson, D. A.; Wilhelm, R. Synlett 2001, 1632-1634.
Abstract: Dilithiation of methoxymethoxyarenetricarbonylchromium complexes by 2.5 equivalents of BuLi and 6 equivalents of (-)-sparteine followed by enantioselective electrophilic quench gave the planar chiral (R)-enantiomers in up to 95% enantiomeric excess. This was the opposite enantiomer obtained under conditions for enantioselective monolithiation with (-)-sparteine/BuLi.
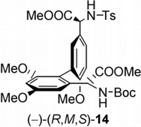
[5] Asymmetric Synthesis of a Fully Protected ent-Actinoidinic Acid. Wilhelm, R.; Widdowson, D. A. Org. Lett. 2001, 3, 3079–3082.
[4] Asymmetric Deprotonation – Substitution of Arenetricarbonylchromium(0) Complexes: Substituent Controlled Lithiation with the Butyllithium-Sparteine System. Wilhelm, R.; Sebhat, I. K.; White, A. J. P.; Williams, D. J.; Widdowson, D. A. Tetrahedron: Asymmetry 2000, 11, 5003-5016.
[3] Palladium Catalyzed Cross-coupling of (Fluoroarene)tricarbonylchromium(0) Complexes. Wilhelm, R.; Widdowson, D. A. J. Chem. Soc., Perkin Trans. 1 2000, 3808-3813.
Abstract: (Fluoroarene)tricarbonylchromium(0) complexes were found to undergo Suzuki and Stille cross-coupling reactions to form functionalised biaryl and styrene complexes in up to 87 and 52% yields, respectively. The Suzuki reactions were optimal with dipalladium tris(dibenzylideneacetone)–trimethylphosphine–caesium carbonate in DME at reflux. The Stille reactions were optimal with dipalladium tris(dibenzylideneacetone)–trimethylphosphine–caesium fluoride in DME at reflux and neither was adversely affected by a methoxy group on the complexed ring. The Suzuki reaction tolerated a chloro group on the arylboronic acid ring but not a bromo group.
[2] Directed Lithiation in Arenetricarbonylchromium(0) Complexes: Assessment of Some Directing Group Specificities and of Electrophilic Quench Efficacies. Sebhat, I. K.; Tan, Y.-L.; Widdowson, D. A.; Wilhelm, R.; White, A. J. P.; Williams, D. J. Tetrahedron 2000, 56, 6121-6134.
Abstract: The synthesis of a series of phenol ethers and 4-triisopropylsilyloxymethylphenol ethers and their η6-tricarbonylchromium(0) complexes are reported. The directed lithiation of these followed by quenching with a series of electrophiles gave access to a wide range of functionalised complexes for use in synthesis.
[1] Palladium Catalyzed Cross-coupling of (Fluoroarene)tricarbonylchromium(0) Complexes. Widdowson, D. A.; Wilhelm, R. Chem. Commun. 1999, 2211-2212.
Abstract: Fluoroarenetricarbonylchromium(0) complexes were found to undergo Suzuki reactions with arylboronic acids to form biaryltricarbonylchromium(0) complexes in the presence of trimethylphosphine/palladium dibenzylideneacetone and caesium carbonate or caesium fluoride.