Macromolecular Crowding
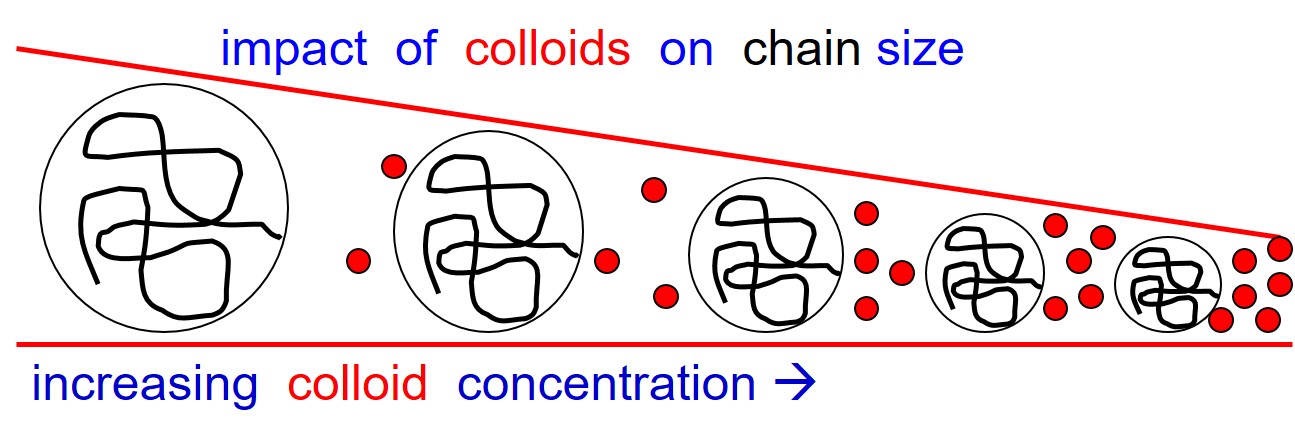
Conformational changes of proteins or DNA as well as the formation of hierarchical structures with proteins are common processes in living cells. Their investigation in vitro is usually based on experimental conditions with properly adjusted pH, salinity, temperature and concentrations of the components directly involved in the respective process. Although such in vitro experiments provide first insights into the mechanisms of the processes under consideration, they totaly neglect the crowded environment in cells, generated by the presence of osmolytes, chaperones and the many other biopolymers. We develop synthetic model systems which mimic biological environments and at the same time reveal distinct aspects of morphological transformations under such crowded environment. One example is the collpase of polystyrene chains in good solvent obsreved after addition of small colloidal particles acting as crowders. Another example is the aggregation of the cationic dyestuff pseudo-isocynanine chloride (PIC) in the absence and presence of various model crowders like Ficoll or poly(ethylene glycol), which takes adavantage of an analogy between many dyestuffs and proteins.
Kramer,T. et al.
Coil dimensions of Polystyrene Chains in Colloid-Polymer Mixtures at the Protein-Limit: A SANS Study
Macromolecules (2005) 38, 9783-9793 DOI: 10.1021/ma051308j
Kramer, T. et al.
Small-Angle Neutron Scattering of dilute Polystyrene Chains at the Protein Limit of a Colloid-Polymer Mixture
J. Chem. Phys. (2005) 123, 014903 DOI: 10.1063/1.1946751
Gomez, D. et al.
On Protein Folding in Crowded Conditions
J. Phys. Chem. Lett. (2019) 10, 7650−7656 DOI: 10.1021/acs.jpclett.9b02642