Antimicrobial Therapies
The global spreading of antibiotic resistance is one of the greatest threats of the 21st century and can be compared to climate change in terms of its severity and effect on the world’s population. In 2019, almost 5 million deaths were associated with drug-resistant bacterial infections. At the same time, however, fewer and fewer new antibiotics are brought to market with yearly FDA approvals steadily decreasing since the 1980s. Without efficient antibiotics, many of the achievements of modern medicine that we today take for granted, such as major surgery, organ transplantation, and cancer chemotherapy, will no longer be available. Therefore, novel and unconventional approaches for the treatment of multidrug-resistant infections are urgently needed. We thus investigate and develop alternative approaches beyond the application of conventional antibiotics to combat drug-resistant bacteria and stop their spreading.
DNA-based nanoantibiotics
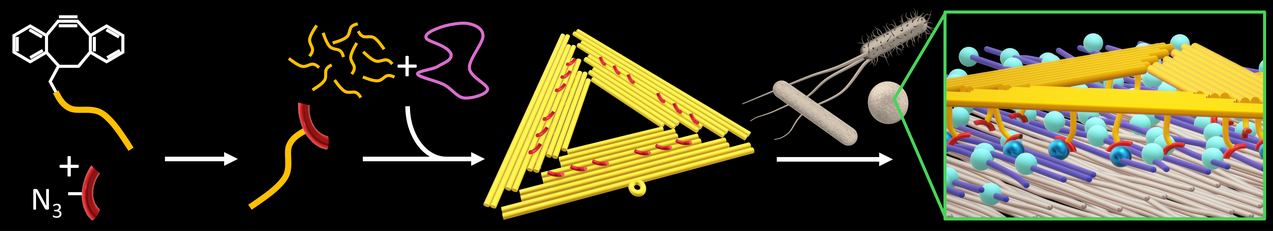
During the last decade, DNA origami nanostructures have become popular and widely employed tools in biomedical research. Compared to conventional organic or inorganic nanoparticles, DNA origami nanostructures have several advantages. Being composed entirely of DNA, they are fully biocompatible, biodegradable, and show very low host toxicity. They can be loaded with numerous therapeutic cargos such as small molecules, enzymes, and nucleic acids via various mechanisms. Additionally, targeting elements can be introduced in a similar manner, enabling the DNA origami nanostructures to specifically bind to the target cells. The vast majority of studies in this research area were traditionally aimed at applications in cancer therapy, whereas the field of infectious diseases has largely been neglected. Therefore, our research explores applications of DNA origami nanostructures in antimicrobial drug delivery. We are particularly interested in their controlled loading with antimicrobial molecules and means for the specific targeting of bacterial pathogens.
Selected publications:
- Vancomycin-Modified DNA Origami Nanostructures for Targeting Bacterial Pathogens, Ö. Coşkuner Leineweber, B.K. Pothineni, N. Schumann, U. Hofmann, C. Möser, D.M. Smith, G. Grundmeier, Y. Zhang, and A. Keller, Small Struct. 6 (2025) e202500246 (Cover Picture)
- Effect of DNA Origami Nanostructures on Bacterial Growth, J.A. Garcia-Diosa, G. Grundmeier, and A. Keller, ChemBioChem 25 (2024), e202400091
Antimicrobial susceptibility testing
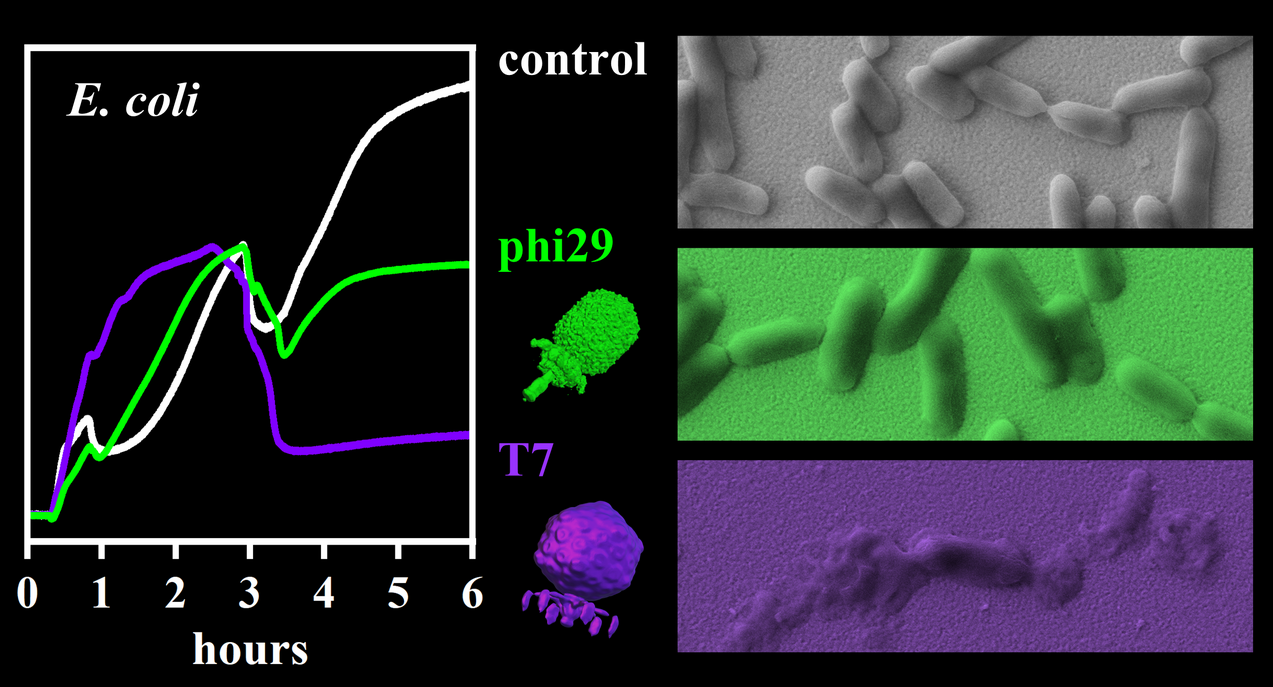
One of the challenges faced in the treatment of multidrug-resistant infections is the accurate and rapid determination of resistance profiles to aid physicians in the selection of the most effective therapeutic options. Microbial response to an antimicrobial agent is evaluated by antimicrobial susceptibility testing (AST). While conventional culture-based AST methods are well-established in diagnostics, they are rather slow. For most clinically important microbes, results are available only after 18 to 48 h. For many nosocomial infections, however, such turnaround times are unacceptably long. Therefore, novel rapid, parallel, and sensitive methods to detect resistances are urgently needed in order to save patient lives, prevent further spreading of infections, and slow down the development of new resistances. We thus explore new approaches based on physicochemical techniques to detect and characterize the response of microbial cells to antimicrobial agents such as small molecules, peptides, and bacteriophages.
Selected publications:
- Monitoring phage infection and lysis of surface-immobilized bacteria by QCM-D, B.K. Pothineni, R. Probst, D. Kiefer, V. Dobretzberger, I. Barišić, G. Grundmeier, and A. Keller, Anal. Bioanal. Chem. 417 (2025) 2143
Antimicrobial photodynamic therapy

Antimicrobial photodynamic therapy is a promising alternative to classical antibiotics-based therapeutic approaches. It uses reactive oxygen species (ROS) that are generated by photosensitizers under irradiation with light of a suitable wavelength. These ROS damage surrounding cells through the non-specific oxidation of various cellular subunits, most importantly the cell membrane. While this non-specific mechanism of action makes the emergence of resistance very unlikely, it can also damage host cells. Therefore, we explore new nanotechnology-based strategies to transport photosensitizers directly to the bacterial cells and protect host tissue. Our current research is focused on the application of DNA nanostructures, which on the one hand can efficiently be loaded with established photosensitizers for targeted delivery, but on the other hand may also scavenge and inactivate the generated ROS.
Selected publications:
- Ion-Dependent Stability of DNA Origami Nanostructures in the Presence of Photo-Generated Reactive Oxygen Species, L. Rabbe, J.A. Garcia-Diosa, G. Grundmeier, and A. Keller, Small Struct. 5 (2024) 2400094 (Frontispiece)
- Highly Efficient Quenching of Singlet Oxygen by DNA Origami Nanostructures, J.A. Garcia-Diosa, G. Grundmeier, and A. Keller, Chem. Eur. J. 30 (2024) e202402057 (Cover Picture)